Use of Sensors in Air Quality Measurements
 The 2B Tech Personal Air Monitor (PAM) contains sensors for measurements of PM1, PM2.5, CO and CO2.
The 2B Tech Personal Air Monitor (PAM) contains sensors for measurements of PM1, PM2.5, CO and CO2.
Over the past few years there has been an enormous surge in interest in the application of low-cost sensors to measurements of air pollutants by educators, citizen scientists and members of various groups interested in air pollution levels in their own communities. This interest has stemmed from a combination of factors that include: development and marketing of low-cost electrochemical sensors for gas-phase species; 2) application of low-cost optical particle counters (OPCs), originally developed for monitoring of HVAC systems, to measurements of ambient particle density and inferred mass concentrations; 3) introduction of the hobbyist microcontroller circuit boards such as the Arduino board; and 4) recent advances in mobile phone app technology that provides for easy display and mapping of data. As a result, dozens of groups, including many university groups, NGOs and small companies, have developed sensor-based devices for measurements of a wide variety of air pollutants. Such devices typically purport to measure various combinations of PM (PM1, PM2.5 & PM10), CO, CO2, O3, NO, NO2, SO2, H2S, VOCs and black carbon. The US EPA has responded to the growing public interest in sensors by developing an Air Sensor Toolbox for Citizen Scientists with an abundance of information about available sensors, how to use them and how to interpret the measurements. Programs to evaluate sensors have been established by both the US EPA (Feinberg et al., 2018; Jiao et al., 2016) and by the Air Quality Sensor Performance Evaluation Center (AQ-SPEC) of California’s South Coast Air Quality Management District.
A majority of the ~50 commercially available sensor packages tested by the EPA and SCAQMD have performed very poorly, with coefficients of determination (R2) values of 0.5 or less, and several sensors having R2 values of ~0.0 (i.e., “random noise generators”). The 2B Tech Personal Ozone Monitor (POM) had the best performance in these independent tests by SCAQMD with measured R2 values of 0.99 in the lab and 1.00 in the field when compared with FEM reference instruments. Of course, the POM is a miniaturized instrument (and a US EPA Federal Equivalent Method) – not a sensor. These independent tests have in some cases led to sensor improvements, and newer versions of some sensor packages have performed better in retesting. Those sensor packages that make use of multiple sensors calibrated using multivariate methods upon co-location with reference methods in the real atmospheric environment perform the best, it is now generally agreed that for most sensors lab calibrations simply do not work; for accurate measurements sensors must be frequently calibrated in the field by sampling real ambient air and compared with co-located reference instruments.
A majority of the ~50 commercially available sensor packages tested by the EPA and SCAQMD have performed very poorly, with coefficients of determination (R2) values of 0.5 or less, and several sensors having R2 values of ~0.0 (i.e., “random noise generators”). The 2B Tech Personal Ozone Monitor (POM) had the best performance in these independent tests by SCAQMD with measured R2 values of 0.99 in the lab and 1.00 in the field when compared with FEM reference instruments. Of course, the POM is a miniaturized instrument (and a US EPA Federal Equivalent Method) – not a sensor. These independent tests have in some cases led to sensor improvements, and newer versions of some sensor packages have performed better in retesting. Those sensor packages that make use of multiple sensors calibrated using multivariate methods upon co-location with reference methods in the real atmospheric environment perform the best, it is now generally agreed that for most sensors lab calibrations simply do not work; for accurate measurements sensors must be frequently calibrated in the field by sampling real ambient air and compared with co-located reference instruments.
Making Sense of Sensors
According to the Merriam Webster dictionary, the definitions of a Sensor and an Instrument are [emphasis ours]:
Sensor: “a device that responds to a physical stimulus (as heat, light, sound, pressure, magnetism, or a particular motion) and transmits a resulting impulse (as for measurement or operating a control).”
Instrument: “a measuring device for determining the present value of a quantity under observation.”
 Students measuring air pollutants along a trek of their own design as part of our AQTreks educational outreach program.
Students measuring air pollutants along a trek of their own design as part of our AQTreks educational outreach program.
It is important to remember that AQ sensors respond to air pollutant in a way similar to our ability to touch an object and respond to whether it is hot or cold or taste a food and give a sweet, bitter, salty, etc. response. Although the electrical signals produced by sensors can be measured with high accuracy, it is often difficult to quantify the concentration of the air pollutant (“determine the present value”) using most low cost sensors. There are several reasons for this, which vary for different types of sensors, as discussed in some detail in the section below. Some of the difficulties in quantifying sensor signals to obtain atmospheric concentrations include:
- Cross sensitivity: a sensor may respond with varying degrees to different chemical species. For example, a NO2 sensor signal may be partly due to NO2, partly due to O3, partly due to SO2, etc.
- Environmental Effects: Sensor response often depends on temperature, pressure and humidity.
- Sensitivity Drift: The response (sensitivity) of the sensor may vary over time. This can be due to depletion of redox species and/or electrolytes in an electrochemical sensor, adsorption of contaminants to solid-state sensors, etc.
- Baseline Drift: The offset (signal in the absence of the analyte being measured) may become positive or negative over time for a variety of reasons such as contamination of active sensor surfaces, accumulation of particles inside a PM sensor, etc.
- Linearity and Dynamic Range: Ideally, a sensor would have a linear response over several orders of magnitude, as do nearly all traditional instruments. Sensors tend to respond linearly over only one to two orders of magnitude, compared to approximately five order of magnitude for the measurement of ozone by UV absorbance, for example. However, the lack of a wide dynamic range is seldom a serious limitation for AQ sensors since the concentration range of interest is not that wide.
- Air Quality Education: Many groups are employing AQ sensors in hands-on educational projects, such as our AQTreks project, that allow K-12 and college students to learn about air pollution (Ellenburg, et al., 2019). Advantages of sensors for this application include low cost, mobility and relatively fast response time. Students can explore where different air pollutants are high or low – for example near or far away from a busy street. Here, learning is facilitated by the air pollution monitoring experience and qualitative data may be acceptable. It is important, however, that students be made aware of the accuracy of their measurements. One way is to compare their results to nearby monitoring stations. The AQTreks mobile app allows students to see the locations of all nearby state and local compliance monitoring stations and their most recent 1-hour averages. Fixed-base stations making use of sensors is valuable education as well. Students can observe variations with time of day (traffic hours, diurnal changes, etc.) and seasonal variations in different pollutant concentrations.
- High Density Sensor Arrays: There are only a handful of monitoring stations dedicated to making accurate measurements of air pollutants in each of the US states, only ~2,000 stations monitor the air breathed by more than 330 million people. Furthermore, these stations are located to provide approximately average air pollutant concentrations in a given region; high concentration areas near pollution sources. The low capital cost of sensor deployments make it possible to fill in the gaps between stations operating highly accurate instruments so that maps of air pollutants can be developed with much higher spatial resolution. This is important, especially in terms of environmental justice, since lower income housing is frequently located close to air pollution sources such as highways, factories and power plants. The use of hundreds of low cost monitoring stations would allow us to better map the distribution of air pollutions throughout a city or region and to better identify air pollution sources. Here, it is important that the calibrations of sensors at individual stations be well maintained, especially in relation to one another, but also in relation to accurate instrumental measurements. This can be accomplished by co-locating sensor packages with State and Local Air Monitoring Stations (SLAMS) before and after deployment and by frequent checking of individual deployed stations using portable instruments and/or calibrators (e.g., Hagan, 2018).
- Mobile Monitoring: Mobile monitoring of air pollutants for high resolution mapping has recently been successfully demonstrated using Google Street Cars (Apke, 2017). Although the measurements of CO, CO2 and black carbon in that study made use of highly accurate traditional instruments, future fleets of monitoring vehicles (delivery trucks, etc.) are likely to make use of AQ sensors because of their small size and low cost. Such sensors will require frequent calibration. One promising approach that 2B Tech is pursuing is the development of AQSync drive-by calibration stations. The AQSync stations, which will be mounted to lamp posts and traffic lights along city streets, will contain miniaturized instruments, including our Model 106 Ozone Monitor, Model 405 nm NO2/NO/NOx Monitor, Black Carbon Photometer, a high quality optical particle counter for PM1 and PM2.5 and highly reliable CO and CO2 sensors. When vehicles carrying sensor packages pass by the AQSync stations, their measurements of air pollutants can be compared to those of accurate instruments.
Types of AQ Sensors
Here we describe the most common sensors currently used in AQ sensor packages and discuss their various limitations and recommendations with respect to their use and interpretation of their measurements.
Particulate Matter (PM) Sensors
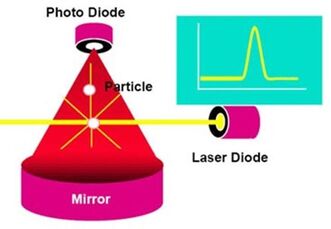 Optical particle counter for measuring the size distribution of particles.
Optical particle counter for measuring the size distribution of particles.
Particulate matter traditionally has been measured by collection on a filter followed by weighing of the filter to determine the weight of the collected particles. By knowing the volume of air sampled through the filter, one can easily calculate the mass concentration of particulate matter in typical units of µg/m3. Different size fractions such as PM10, PM2.5 and PM1 (mass concentrations of particles having diameters less than 10, 2.5 or 1 µm, respectively) are determined by use of impactors to limit the size of particles reaching the filters. This gravimetric method comprises the EPA Federal Reference Method for PM10 and PM2.5 but provides a very slow response time (hours to days) and is very labor intensive.
In order to increase time resolution and reduce labor, automated methods have been developed in recent years. Perhaps the most widely used automated method is the Beta Attenuation Monitor (BAM), which measures the attenuation of low energy beta particles, emanating from a 14C radioactive source, that pass through a filter medium that continuously collects atmospheric particles. This provides a relatively good mass concentration measurement on time scales of 15 min to 1 hr; however, the mass attenuation coefficient of beta particles varies significantly with atomic number of the attenuating material. For this reason, BAMs must occasionally be calibrated against the gravimetric method to account for variations in the elemental composition of the aerosol being measured.
The optical particle counter (OPC) provides an automated method with very fast time response for determining PM mass concentrations. In this method, particles pass through a laser beam and the scattered light is detected as pulses by a light detector (photomultiplier tube or photodiode) usually placed at right angles to the laser beam. The recorded light flashes are binned according to intensity, and particles of known diameter are used to calibrate the bins to correspond to size ranges. From the counts in the individual size bins and an assumed particle density, it is then possible to calculate the mass of particles passing through the laser beam during a relatively short period of time, usually one or more seconds. Like the BAM method, this approach must occasionally be calibrated against a gravimetric standard since both the scattering coefficient and particle density depend on particle composition. Advantages of OPC monitors include high sensitivity and very fast response times of typically seconds.
FEM instruments based on optical particle counting are relatively expensive. In recent years, inexpensive sensors based on optical particle counting (e.g., Plantower) have been developed, primarily for the HVAC industry. Many companies have now applied low-cost (~20 USD) OPC sensors to measurements of PM with considerable success. The most common of these is PurpleAir which combines two Plantower PMS 5003 particle sensors (redundant measurements for fault detection) with an Arduino microcontroller, both of which are mounted in a PVC pipe cap with a metal bracket attached for mounting. The PurpleAir PA-II requires a power source, and data are provided via a wifi internet connection. This plug-and-play device sells for ~200 USD. The specified counting efficiency is 50% for 0.3 µm and 98% for ≥ 0.5 µm particle diameter. A field evaluation by AQ-SPEC gave R2 values of 0.96-0.98 for PM1, 0.93-0.97 for PM2.5 and 0.66 to 0.70 for PM10 when capered to a GRIMM (also an OPC) and BAM FEM monitors. With the exception of PM10, for which it was noted that PurpleAir PA-II “nodes did not always follow the PM10 concentration change,” this is remarkable correlation. We have found similar good correlations of the Plantower PMS 5003 with an FEM optical particle counter for PM2.5, which is used in both our PAM and CAM but poor correlation with PM10. The lack of correlation with PM10 is to be expected for these simple devices because of the difficulty in sampling large particles, which are easily lost to impaction with surfaces in the flow path. Accurate measurements of PM10 require specially designed inlet systems not available to small sensor packages, so PM10 measurements using OPC sensors are qualitative at best.
Because particles take up water, changing their size distribution based on relative humidity, it has been found that a humidity correction is required to obtain good agreement with FEM monitors under varying humidity conditions. The correction factor found by Zheng et al. (2018) at the Raleigh-Durham Research Triangle monitoring site is:
In order to increase time resolution and reduce labor, automated methods have been developed in recent years. Perhaps the most widely used automated method is the Beta Attenuation Monitor (BAM), which measures the attenuation of low energy beta particles, emanating from a 14C radioactive source, that pass through a filter medium that continuously collects atmospheric particles. This provides a relatively good mass concentration measurement on time scales of 15 min to 1 hr; however, the mass attenuation coefficient of beta particles varies significantly with atomic number of the attenuating material. For this reason, BAMs must occasionally be calibrated against the gravimetric method to account for variations in the elemental composition of the aerosol being measured.
The optical particle counter (OPC) provides an automated method with very fast time response for determining PM mass concentrations. In this method, particles pass through a laser beam and the scattered light is detected as pulses by a light detector (photomultiplier tube or photodiode) usually placed at right angles to the laser beam. The recorded light flashes are binned according to intensity, and particles of known diameter are used to calibrate the bins to correspond to size ranges. From the counts in the individual size bins and an assumed particle density, it is then possible to calculate the mass of particles passing through the laser beam during a relatively short period of time, usually one or more seconds. Like the BAM method, this approach must occasionally be calibrated against a gravimetric standard since both the scattering coefficient and particle density depend on particle composition. Advantages of OPC monitors include high sensitivity and very fast response times of typically seconds.
FEM instruments based on optical particle counting are relatively expensive. In recent years, inexpensive sensors based on optical particle counting (e.g., Plantower) have been developed, primarily for the HVAC industry. Many companies have now applied low-cost (~20 USD) OPC sensors to measurements of PM with considerable success. The most common of these is PurpleAir which combines two Plantower PMS 5003 particle sensors (redundant measurements for fault detection) with an Arduino microcontroller, both of which are mounted in a PVC pipe cap with a metal bracket attached for mounting. The PurpleAir PA-II requires a power source, and data are provided via a wifi internet connection. This plug-and-play device sells for ~200 USD. The specified counting efficiency is 50% for 0.3 µm and 98% for ≥ 0.5 µm particle diameter. A field evaluation by AQ-SPEC gave R2 values of 0.96-0.98 for PM1, 0.93-0.97 for PM2.5 and 0.66 to 0.70 for PM10 when capered to a GRIMM (also an OPC) and BAM FEM monitors. With the exception of PM10, for which it was noted that PurpleAir PA-II “nodes did not always follow the PM10 concentration change,” this is remarkable correlation. We have found similar good correlations of the Plantower PMS 5003 with an FEM optical particle counter for PM2.5, which is used in both our PAM and CAM but poor correlation with PM10. The lack of correlation with PM10 is to be expected for these simple devices because of the difficulty in sampling large particles, which are easily lost to impaction with surfaces in the flow path. Accurate measurements of PM10 require specially designed inlet systems not available to small sensor packages, so PM10 measurements using OPC sensors are qualitative at best.
Because particles take up water, changing their size distribution based on relative humidity, it has been found that a humidity correction is required to obtain good agreement with FEM monitors under varying humidity conditions. The correction factor found by Zheng et al. (2018) at the Raleigh-Durham Research Triangle monitoring site is:
where RH is the relative humidity (as a fraction, not percent), a = 1.19 and b = 0.119. The raw data are corrected by dividing the measured PM value by the correction factor, CF. Correction factors are likely to be different at different sites due to different mixes of aerosol types, but the above equation can serve as a first-order approximation. Zheng et al. (2018) identified a temperature correction as well, but the correction is small with a maximum correction of ~4%, and applying this correction is not well justified since its application sometimes creates more, rather than less, variance in the correlation with FEM monitors.
Because OPCs measure light scattering rather than mass, it is necessary to co-locate the sensors with a FRM or gravimetrically calibrated FEM method for a few days to weeks in order to calibrate for the local aerosol average composition. And, ideally, this should be done a few times per year to account for seasonal changes in the aerosol mix. Of course, this is true for the BAM and OPC FEM methods as well.
Because OPCs measure light scattering rather than mass, it is necessary to co-locate the sensors with a FRM or gravimetrically calibrated FEM method for a few days to weeks in order to calibrate for the local aerosol average composition. And, ideally, this should be done a few times per year to account for seasonal changes in the aerosol mix. Of course, this is true for the BAM and OPC FEM methods as well.
Electrochemical Sensors
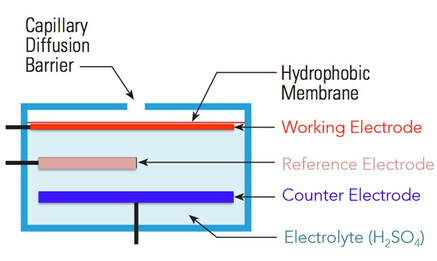 Schematic diagram of a 3-electrode electrochemical sensor.
Schematic diagram of a 3-electrode electrochemical sensor.
Electrochemical sensors have long been used for measurements of air pollutants to protect the health of and safety of industrial workers. For those industrial hygiene applications, ppm sensitivity is adequate, and the portable monitors are primarily used as alarms to alert individuals of dangerous gas leaks. Such sensors have been refined in recent years by AlphaSense and others to provide adequate sensitivity at the ppb level for a wide range of gaseous air pollutants such as NO, NO2, O3, SO2 CO and H2S. Electrochemical sensors for air pollutants are based on amperometry – the measurement of a current produced upon oxidation or reduction of the air pollutant, as shown in Fig. 2. Components of air diffuse into the sensor via a capillary orifice or inert hydrophobic membrane such as porous PTFE where they dissolve into an electrolyte solution such as sulfuric acid. The working electrode potential is poised positive or negative relative to the reference (e.g., Ag/AgCl) in order to promote reduction or oxidation, respectively, of the analyte at the working electrode. The opposite process, oxidation or reduction, occurs at the counter electrode. The purpose of a 3-electrode system is to maintain a constant potential at the working electrode relative to the reference electrode regardless of the amount of current flowing. This is achieved by use of a potentiostat that maintains a constant potential between the working electrode and reference electrode while allowing the potential of the counter electrode to float. The Alphasense sensors actually use a more sophistical 4-electrode system to further improve the precision and accuracy of the Faradaic current (electrochemical current due to oxidation and reduction) measurement.
Species detected by oxidation (removal of electrons) include CO, H2S, NO and SO2, and species detected by reduction include O3 and NO2. Selectivity can be achieved by choice of the working electrode potential in combination with choice of an electrode surface (a catalyst that reduces the over potential) that is selective for oxidation or reduction of the species of interest relative to other species that, based on thermodynamics, can be oxidized or reduced at the same potential. Cross sensitivity is a huge problem for some species. For example the Ox sensor responds approximately equally to NO2 and O3, and the NO2 sensor has an approximately equal and opposite responses to NO2 and SO2. The NO2 sensor achieves selectivity against O3 by incorporating a catalyst prior to the working electrode to decompose O3 to O2. Ozone can then be obtained by difference between the signals from Ox and NO2 sensors, but this approach to ozone measurement is subject to considerable error.
Of all the electrochemical sensors, the one for CO is the most robust, and provides reliable measurements when properly calibrated. This is partly due to the fact that CO is a reduced species, for which there are few potential interferences, and is often present at ppm levels, rather than low ppb levels, in the atmosphere. Oxidation or reduction at the working electrode is balanced by the opposite process at the counter electrode. For example, when CO is oxidized at the working electrode to produce CO2, oxygen is reduced at the counter electrode to produce water:
Oxidation at working electrode: CO + H2O → CO2 + 2 H+ + 2 e-
Reduction at counter electrode: ½ O2 + 2 H+ + 2 e- → H2O
__________________________
Net Reaction: CO + ½ O2 → CO2
When reduction occurs at the working electrode (O3, NO2), oxygen is produced at the counter electrode. For example, in the reduction of O3:
Reduction at working electrode: O3 + 2 H+ + 2 e- → O2 + H2O
Oxidation at counter electrode: H2O → 2 H+ ½ O2 + 2e-
_________________________
Net Reaction: O3 → 3/2 O2
Transport of gaseous analytes to the working electrode is ideally by diffusion, which is temperature and pressure dependent. Care must be taken to have a constant flow rate over the sensor membrane surface in order to maintain a constant thickness of the boundary layer or "diffusion layer" through which the CO or other analyte must diffuse. Higher flow rates over the membrane result in a thinner diffusion layer, shorter diffusion time and greater signal. Therefore, variations in flow rate result in variations in sensitivity to the same gas concentration.
Compared to optical instruments, the main advantages of electrochemical sensors include their small size and weight, low power requirements and low cost. The disadvantage is primarily that of accuracy due to cross sensitivities and changes in calibration (both offset and sensitivity) due to changes in environmental factors of temperature, pressure and humidity and adsorption on the working electrode surface by other chemical species in air. Approximate corrections can be made for environmental factors, but changes in sensitivity due to contamination of the membrane or “poisoning” of the electrode surface are unpredictable, making frequent calibration necessary. Long-term drift in sensitivity results from a variety of factors including slow loss of electrolyte due to evaporation. Cross sensitivities can be accounted by multivariate analysis if multiple species are measured, including all mutually inferring species, but such corrections introduce uncertainty in the measurements as well.
Species detected by oxidation (removal of electrons) include CO, H2S, NO and SO2, and species detected by reduction include O3 and NO2. Selectivity can be achieved by choice of the working electrode potential in combination with choice of an electrode surface (a catalyst that reduces the over potential) that is selective for oxidation or reduction of the species of interest relative to other species that, based on thermodynamics, can be oxidized or reduced at the same potential. Cross sensitivity is a huge problem for some species. For example the Ox sensor responds approximately equally to NO2 and O3, and the NO2 sensor has an approximately equal and opposite responses to NO2 and SO2. The NO2 sensor achieves selectivity against O3 by incorporating a catalyst prior to the working electrode to decompose O3 to O2. Ozone can then be obtained by difference between the signals from Ox and NO2 sensors, but this approach to ozone measurement is subject to considerable error.
Of all the electrochemical sensors, the one for CO is the most robust, and provides reliable measurements when properly calibrated. This is partly due to the fact that CO is a reduced species, for which there are few potential interferences, and is often present at ppm levels, rather than low ppb levels, in the atmosphere. Oxidation or reduction at the working electrode is balanced by the opposite process at the counter electrode. For example, when CO is oxidized at the working electrode to produce CO2, oxygen is reduced at the counter electrode to produce water:
Oxidation at working electrode: CO + H2O → CO2 + 2 H+ + 2 e-
Reduction at counter electrode: ½ O2 + 2 H+ + 2 e- → H2O
__________________________
Net Reaction: CO + ½ O2 → CO2
When reduction occurs at the working electrode (O3, NO2), oxygen is produced at the counter electrode. For example, in the reduction of O3:
Reduction at working electrode: O3 + 2 H+ + 2 e- → O2 + H2O
Oxidation at counter electrode: H2O → 2 H+ ½ O2 + 2e-
_________________________
Net Reaction: O3 → 3/2 O2
Transport of gaseous analytes to the working electrode is ideally by diffusion, which is temperature and pressure dependent. Care must be taken to have a constant flow rate over the sensor membrane surface in order to maintain a constant thickness of the boundary layer or "diffusion layer" through which the CO or other analyte must diffuse. Higher flow rates over the membrane result in a thinner diffusion layer, shorter diffusion time and greater signal. Therefore, variations in flow rate result in variations in sensitivity to the same gas concentration.
Compared to optical instruments, the main advantages of electrochemical sensors include their small size and weight, low power requirements and low cost. The disadvantage is primarily that of accuracy due to cross sensitivities and changes in calibration (both offset and sensitivity) due to changes in environmental factors of temperature, pressure and humidity and adsorption on the working electrode surface by other chemical species in air. Approximate corrections can be made for environmental factors, but changes in sensitivity due to contamination of the membrane or “poisoning” of the electrode surface are unpredictable, making frequent calibration necessary. Long-term drift in sensitivity results from a variety of factors including slow loss of electrolyte due to evaporation. Cross sensitivities can be accounted by multivariate analysis if multiple species are measured, including all mutually inferring species, but such corrections introduce uncertainty in the measurements as well.
NDIR Sensors
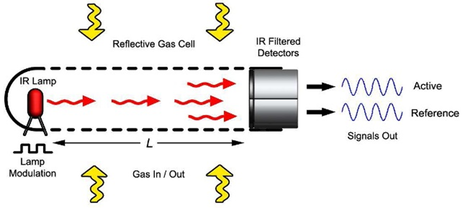 Schematic diagram of a NDIR sensor.
Schematic diagram of a NDIR sensor.
Non-dispersive infrared (NDIR) sensors may be used to measure variations in CO2 both indoors and in the ambient atmosphere where it is present at ~415 ppm (approximate global average in late 2020 and increasing by 2-3 ppm/year) in the absence of local air pollution. Levels indoors can be as much as a few thousand ppm due to human respiration. NDIR sensors for CO2 are based on absorbance of the molecule in the gas phase at an infrared wavelength of ~4.26 µm. The sensor has a built in light emitting diode (LED) as the light source and an infrared detector such as a thermopile or pyroelectric detector to measure the light intensity. While NDIR instruments make use of CO2 gas sealed in one half of a rotating filter wheel to rapidly block and pass wavelengths absorbed by CO2, NDIR sensors, developed originally for HVAC systems where high accuracy is not required, make use of a narrow bandpass filter to limit the wavelength range detected. A second reference detector with a slightly offset bandpass filter serves as a reference for ratioing out fluctuations in the infrared source. In order to reduce baseline noise, the light source is modulated. Corrections are usually made within the sensor for variations in temperature and pressure, which affect both the sensitivity due to changes in the vibrational-rotational line widths of the CO2 absorption spectrum and the air density needed to calculate a mixing ratio in ppm. Also, correction must be made for non-linearity due to Beer-Lambert Law deviations due to the band width of the light source being wider than the absorption band, which is composed of narrow lines. Because of these factors in combination with a short path length, precision and accuracy are generally limited to a few tens of ppm. NDIR sensors may be thought of as highly miniaturized optical instruments. They tend to be much less sensitive to baseline and sensitivity drift than electrochemical and HMOS sensors because the measurement is made optically in the gas phase with no requirement for a gas/liquid or gas/solid interface.
Heated Metal Oxide Semiconductor (HMOS) Sensors
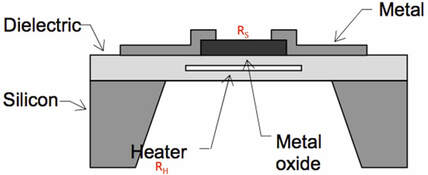 Schematic diagram of a HMOS sensor.
Schematic diagram of a HMOS sensor.
Heated Metal Oxide Semiconductor (HMOS) sensors have been developed for species such as ozone, H2S, CO and volatile organic compounds (VOCs). Of these, ozone has been used for more than a decade in portable ozone-monitoring devices manufactured by Aeroqual and Eco Sensors. HMOS sensors based on both n- and p-type semiconductors have been developed. Sensors are heated to typically 400 °C to promote electrons to the conduction band and reduce resistance. Adsorption and/or reaction of chemical species to the surface within a porous layer increases the electrical resistance, which is measured. In some HMOS sensors, the temperature is periodically pulsed to ~525 °C to drive off adsorbed species and/or re-oxidize the surface to prevent downward sensitivity drift. Selectivity for different gases is achieved by proprietary doping and surface treatment of the semiconductor. With the exception of ozone, which can be measured down to low ppb levels, HMOS sensors are currently only useful at levels of 1-100 ppm and generally useful only in industrial applications where such high concentrations are encountered. Electrochemical sensors are much more sensitive than HMOS sensors for ambient CO levels, for example. HMOS sensors can be used for indoor air measurements of VOCs but lack adequate sensitivity for ambient outdoor measurements. Although more expensive, photoionization detectors (PIDs), are much more sensitive than HMOS detectors for VOCs.
References
Apte, J.S., Messier, K.P., Gani, S., Brauer, M., Kirchstetter, T.W., Lunden, M.M., Marshall, J.D., Portier, C.J., Vermeulen, R.C.H. and Hamburg, S.P. (2017) High-resolution air pollution mapping with Google Street View cars: Exploiting big data, Environmental Science & Technology, 51, 6999–7008. Direct Link
J.A. Ellenburg, C.J. Williford, S.L. Rodriguez, P.C. Andersen, A.A. Turnipseed, C.A. Ennis, K.A. Basman, J.M. Hatz, J.C. Prince, D.H. Meyers, D.J. Kopala, M.J. Samon, K.J. Jaspers, B.J. Latham, B.J. Carpenter and J.W. Birks (2019) Global Ozone (GO3) Project and AQTreks: Use of evolving technologies by students and citizen scientists to monitor air pollutants, Atmospheric Environment: X, 4, 100,048, 1-16 (2019). Direct Link
Feinberg, S., Williams, R., Hagler, G.S.W., Rickard, J., Brown, R., Garver, D., Harshfield, G., Stauffer, P., Mattson, E., Judge, R. and Garvey, S. (2018) Long-term evaluation of air sensor technology under ambient conditions in Denver, Colorado, Atmospheric Measurement Techniques, 11, 4605–4615. Direct Link
Hagan, D.H., Isaacman-VanWertz, G., Franklin, J. P., Wallace, L.M.M., Kocar, B.D., Heald, C.L. and Kroll, J.H. (2018) Calibration and assessment of electrochemical air quality sensors by co-location with regulatory-grade instruments, Atmospheric Measurement Techniques, 11, 315–328. Direct Link
Jiao, W., Hagler, G., Williams, R., Sharpe, R., Brown, R., Garver, D., Judge, R., Caudill, M., Rickard, J., Davis, M., Weinstock, L., Zimmer-Dauphinee, S. and Buckley, K. (2016) Community Air Sensor Network, (CAIRSENSE) project: Evaluation of low-cost sensor performance in a suburban environment in the southeastern United States, Atmospheric Measurement Techniques, 9, 5281–5292. Direct Link
Zheng, T., Bergin, M.H., Johnson, K.K., Tripathi, S.N., Shirodkar, S., Landis, R. Sutaria, R. D.E. Carlson (2018) Field evaluation of low-cost particulate matter sensors in high-and low-concentration environments, Atmospheric Measurement Techniques, 11, 4823-4846. Direct Link
J.A. Ellenburg, C.J. Williford, S.L. Rodriguez, P.C. Andersen, A.A. Turnipseed, C.A. Ennis, K.A. Basman, J.M. Hatz, J.C. Prince, D.H. Meyers, D.J. Kopala, M.J. Samon, K.J. Jaspers, B.J. Latham, B.J. Carpenter and J.W. Birks (2019) Global Ozone (GO3) Project and AQTreks: Use of evolving technologies by students and citizen scientists to monitor air pollutants, Atmospheric Environment: X, 4, 100,048, 1-16 (2019). Direct Link
Feinberg, S., Williams, R., Hagler, G.S.W., Rickard, J., Brown, R., Garver, D., Harshfield, G., Stauffer, P., Mattson, E., Judge, R. and Garvey, S. (2018) Long-term evaluation of air sensor technology under ambient conditions in Denver, Colorado, Atmospheric Measurement Techniques, 11, 4605–4615. Direct Link
Hagan, D.H., Isaacman-VanWertz, G., Franklin, J. P., Wallace, L.M.M., Kocar, B.D., Heald, C.L. and Kroll, J.H. (2018) Calibration and assessment of electrochemical air quality sensors by co-location with regulatory-grade instruments, Atmospheric Measurement Techniques, 11, 315–328. Direct Link
Jiao, W., Hagler, G., Williams, R., Sharpe, R., Brown, R., Garver, D., Judge, R., Caudill, M., Rickard, J., Davis, M., Weinstock, L., Zimmer-Dauphinee, S. and Buckley, K. (2016) Community Air Sensor Network, (CAIRSENSE) project: Evaluation of low-cost sensor performance in a suburban environment in the southeastern United States, Atmospheric Measurement Techniques, 9, 5281–5292. Direct Link
Zheng, T., Bergin, M.H., Johnson, K.K., Tripathi, S.N., Shirodkar, S., Landis, R. Sutaria, R. D.E. Carlson (2018) Field evaluation of low-cost particulate matter sensors in high-and low-concentration environments, Atmospheric Measurement Techniques, 11, 4823-4846. Direct Link

